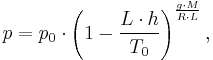Atmospheric pressure
Atmospheric pressure is the force per unit area exerted into a surface by the weight of air above that surface in the atmosphere of Earth (or that of another planet). In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point. Low-pressure areas have less atmospheric mass above their location, whereas high-pressure areas have more atmospheric mass above their location. Likewise, as elevation increases, there is less overlying atmospheric mass, so that pressure decreases with increasing elevation. A column of air one square centimeter in cross-section, measured from sea level to the top of the atmosphere, has a mass of about a kilogram and a weight of about 9.8 N (2.2 lbs force) (and a column one square inch in cross-section would weigh about 14 lbs force (63 N)).
Contents |
Standard atmospheric pressure
The standard atmosphere (symbol: atm) is a unit of pressure and is defined as being equal to 101.325 kPa.[1] The following units are equivalent, but only to the number of decimal places displayed: 760 mmHg (torr), 29.92 inHg, 14.696 psi, 1013.25 millibars/hectopascal. One standard is standard pressure used for pneumatic fluid power (ISO R554), and in the aerospace (ISO 2533) and petroleum (ISO 5024) industries.
In 1971, the International Union of Pure and Applied Chemistry (IUPAC) said that for the purposes of specifying the properties of substances, "the standard pressure" should be defined as precisely 100 kPa (≈750.01 torr) or 29.53 inHg rather than the 101.325 kPa value of “one standard atmosphere”.[2] This value is used as the standard pressure for the compressor and the pneumatic tool industries (ISO 2787).[3] (See also Standard temperature and pressure.) In the United States, compressed air flow is often measured in "standard cubic feet" per unit of time, where the "standard" means the equivalent quantity of air at standard temperature and pressure. For every 1,000 feet you ascend, the atmospheric pressure decreases by about 4%. However, this standard atmosphere is defined slightly differently: temperature = 20 °C (68 °F), air density = 1.225 kg/m³ (0.0765 lb/cu ft), altitude = sea level, and relative humidity = 20%. In the air conditioner industry, the standard is often temperature = 0 °C (32 °F) instead. For natural gas, the Gas Processors Association (GPA) specifies a standard temperature of 60 °F (15.6 °C), but allows a variety of "base" pressures, including 14.65 psi (101.0 kPa), 14.656 psi (101.05 kPa), 14.73 psi (101.6 kPa) and 15.025 psi (103.59 kPa).[4] For a given "base" pressure, the higher the air pressure, the colder it is; the lower the air pressure, the warmer it is.
Mean sea level pressure
Mean sea level pressure (MSLP) is the pressure at sea level or (when measured at a given elevation on land) the station pressure reduced to sea level assuming an isothermal layer at the station temperature.
This is the pressure normally given in weather reports on radio, television, and newspapers or on the Internet. When barometers in the home are set to match the local weather reports, they measure pressure reduced to sea level, not the actual local atmospheric pressure. See Altimeter (barometer vs. absolute).
The reduction to sea level means that the normal range of fluctuations in pressure is the same for everyone. The pressures that are considered high pressure or low pressure do not depend on geographical location. This makes isobars on a weather map meaningful and useful tools.
The altimeter setting in aviation, set either QNH or QFE, is another atmospheric pressure reduced to sea level, but the method of making this reduction differs slightly.
- QNH
- The barometric altimeter setting that will cause the altimeter to read airfield elevation when on the airfield. In ISA temperature conditions the altimeter will read altitude above mean sea level in the vicinity of the airfield
- QFE
- The barometric altimeter setting that will cause an altimeter to read zero when at the reference datum of a particular airfield (in general, a runway threshold). In ISA temperature conditions the altimeter will read height above the datum in the vicinity of the airfield.
QFE and QNH are arbitrary Q codes rather than abbreviations, but the mnemonics "Nautical Height" (for QNH) and "Field Elevation" (for QFE) are often used by pilots to distinguish them.
Average sea-level pressure is 101.325 kPa (1013.25 mbar, or hPa) or 29.92 inches of mercury (inHg) or 760 millimeters (mmHg). In aviation weather reports (METAR), QNH is transmitted around the world in millibars or hectopascals (1 millibar = 1 hectopascal), except in the United States, Canada, and Colombia where it is reported in inches (to two decimal places) of mercury. (The United States and Canada also report sea level pressure SLP, which is reduced to sea level by a different method, in the remarks section, not an internationally transmitted part of the code, in hectopascals or millibars.[5] However, in Canada's public weather reports, sea level pressure is instead reported in kilopascals [1], while Environment Canada's standard unit of pressure is the same [2] [3].) In the weather code, three digits are all that is needed; decimal points and the one or two most significant digits are omitted: 1013.2 mbar or 101.32 kPa is transmitted as 132; 1000.0 mbar or 100.00 kPa is transmitted as 000; 998.7 mbar or 99.87 kPa is transmitted as 987; etc. The highest sea-level pressure on Earth occurs in Siberia, where the Siberian High often attains a sea-level pressure above 1050.0 mbar. The lowest measurable sea-level pressure is found at the centers of tropical cyclones and tornadoes.
Altitude atmospheric pressure variation
Pressure varies smoothly from the Earth's surface to the top of the mesosphere. Although the pressure changes with the weather, NASA has averaged the conditions for all parts of the earth year-round. As altitude increases, atmospheric pressure decreases. One can calculate the atmospheric pressure at a given altitude.[6] Temperature and humidity also affect the atmospheric pressure, and it is necessary to know these to compute an accurate figure. The graph at right was developed for a temperature of 15 °C and a relative humidity of 0%.
The equation relating atmospheric pressure p to altitude h and other parameters is
where the constant parameters are as described below:
| Parameter | Description | Value |
|---|---|---|
| p0 | sea level standard atmospheric pressure | 101325 Pa |
| L | temperature lapse rate | 0.0065 K/m |
| T0 | sea level standard temperature | 288.15 K |
| g | Earth-surface gravitational acceleration | 9.80665 m/s2 |
| M | molar mass of dry air | 0.0289644 kg/mol |
| R | universal gas constant | 8.31447 J/(mol•K) |
Local atmospheric pressure variation
Atmospheric pressure varies widely on Earth, and these changes are important in studying weather and climate. See pressure system for the effects of air pressure variations on weather.
Atmospheric pressure shows a diurnal or semidiurnal (twice-daily) cycle caused by global atmospheric tides. This effect is strongest in tropical zones, with amplitude of a few millibars, and almost zero in polar areas. These variations have two superimposed cycles, a circadian (24 h) cycle and semi-circadian (12 h) cycle.
Atmospheric pressure records
The highest barometric pressure ever recorded on Earth was 1,085.7 hectopascals (32.06 inHg) measured in Tonsontsengel, Mongolia on 19 December 2001.[7] The lowest non-tornadic atmospheric pressure ever measured was 870 hPa (25.69 inches), set on 12 October 1979, during Typhoon Tip in the western Pacific Ocean. The measurement was based on an instrumental observation made from a reconnaissance aircraft.[8]
Atmospheric pressure based on height of water
Atmospheric pressure is often measured with a mercury barometer, and a height of approximately 760 millimetres (30 in) of mercury is often used to illustrate (and measure) atmospheric pressure. However, since mercury is not a substance that humans commonly come in contact with, water often provides a more intuitive way to visualize the pressure of one atmosphere.
One atmosphere (100 kPa or 14.7 psi) is the amount of pressure that can lift water approximately 10.3 m (34 ft). Thus, a diver 10.3 m underwater experiences a pressure of about 2 atmospheres (1 atm of air plus 1 atm of water). This is also the maximum height to which a column of water can be drawn up by suction.
Low pressures such as natural gas lines are sometimes specified in inches of water, typically written as w.c. (water column) or W.G. (inches water gauge). A typical gas using residential appliance is rated for a maximum of 14 w.c., which is approximately 35 hPa.
In general, non-professional barometers are aneroid barometers or strain gauge based. See pressure measurement for a description of barometers.
Boiling point of water
Water boils at about 100 °C (212 °F) at standard atmospheric pressure. The boiling point is the temperature at which the vapor pressure is equal to the atmospheric pressure around the water.[9] Because of this, the boiling point of water is lower at lower pressure and higher at higher pressure. This is why baking at elevations more than 3,500 ft (1,100 m) above sea level requires adjustments to recipes.[10] A rough approximation of elevation can be obtained by measuring the temperature at which water boils; in the mid-19th century, this method was used by explorers.[11]
See also
- Atmosphere (unit)
- Barometric formula
- Barotrauma physical damage to body tissues caused by a difference in pressure between an air space inside or beside the body and the surrounding gas or liquid.
- International Standard Atmosphere - a tabulation of typical variation of principal thermodynamic variables of the atmosphere (pressure, density, temperature etc.) with altitude, at mid latitudes.
- NRLMSISE-00
- Plenum
- Subtropical high belts
- Cabin pressurization
References
- ^ International Civil Aviation Organization, Manual of the ICAO Standard Atmosphere, Doc 7488-CD, Third Edition, 1993, ISBN 92-9194-004-6.
- ^ IUPAC.org, Publications, Standard Pressure (20 kB PDF)
- ^ Compressor.co.za, May 2003 Newsletter
- ^ GPA Standard 2172-09, §3.3
- ^ Sample METAR of CYVR Nav Canada
- ^ A quick derivation relating altitude to air pressure by Portland State Aerospace Society, 2004, accessed 05032011
- ^ Christopher C. Burt (2004). Extreme Weather (1 ed.). Twin Age Ltd.. p. 234. ISBN 0393326586.
- ^ Chris Landsea (2010-04-21). "Subject: E1), Which is the most intense tropical cyclone on record?". Atlantic Oceanographic and Meteorological Laboratory. http://www.aoml.noaa.gov/hrd/tcfaq/E1.html. Retrieved 2010-11-23.
- ^ Vapour Pressure
- ^ Crisco - Articles & Tips - Cooking Tips - High Altitude Cooking
- ^ Berberan-Santos, M. N.; Bodunov, E. N.; Pogliani, L. (1997). "On the barometric formula". American Journal of Physics 65 (5): 404–412. Bibcode 1997AmJPh..65..404B. doi:10.1119/1.18555
External links
- How Atmospheric Pressure Affects Objects (Audio slideshow from the National High Magnetic Field Laboratory)
- NASA page on the 1976 Standard Atmosphere
- Source code and equations for the 1976 Standard Atmosphere
- A mathematical model of the 1976 U.S. Standard Atmosphere
- Calculator using multiple units and properties for the 1976 Standard Atmosphere
- Calculator giving standard air pressure at a specified altitude, or altitude at which a pressure would be standard
- Some of the effects of air pressure
- Atmospheric calculator and Geometric to Pressure altitude converter
Experiments
- Movies on atmospheric pressure experiments from Georgia State University's HyperPhysics website - requires QuickTime
- Test showing a can being crushed after boiling water inside it, then moving it into a tub of ice cold water.
|
|||||||||||||||||